


Benefit: risk assessment is an important term in pharmacovigilance, it is something that all pharmacovigilance professionals should be thinking about on a daily basis. This article outlines the importance of benefit: risk in pharmacovigilance and how the practice has developed, as well as when and how we should make benefit: risk assessments.
The benefit: risk balance is a fundamental aspect of a medicinal product’s profile throughout its life cycle. It is the underlying basis of regulatory decision-making. Initially, it determines whether the competent authorities can authorise a medicine1, as well as establishing whether additional risk minimisation measures are required to be outlined in the risk management plan (RMP)2. Once authorised, benefit: risk is continually assessed, for example during signal detection, periodic benefit: risk assessment reports (PBEREs)/periodic safety update reports (PSURs) at milestones of RMPs and during renewal applications. Post-licencing, particularly, the assessment is aimed at reducing adverse drug reactions (ADRs). To envisage the impact of ADRs on patients, Bouvy et al3 performed a review of all epidemiological studies quantifying ADRs in a European setting between January 2000 and September 2014. They found that on average, the ADR occurrence rate at hospital admission was 3.6%, rising to 10.1% during hospitalisation. Shockingly, they found that estimates suggested approximately 0.25% (or 1 in 400 hospitalised patients) will die as a result of an ADR during their stay in a European hospital. This demonstrates the importance of benefit: risk assessment, to minimise the numbers of ADRs, whilst maximising the benefits and ensuring that patients who will benefit from the medicine are not prevented from receiving it.
But how is benefit: risk assessed?
In 1998, the Council for International Organizations of Medical Sciences (CIOMs) Working Group IV developed a guidance document “Benefit-risk balance for marketed drugs: Evaluating Safety Signals”4. This comprised of a structured guidance on assessing the balance between benefits and risks of marketed products; intended for when a newly established or suspected major safety problem emerges. Ten years later, the Committee for Medicinal Products for Human Use (CHMP) published a report5 and reflection paper6 on benefit-risk assessment models and methods. These focussed on how to improve the methodology, transparency, consistency and communication of the benefit: risk assessment and a review of specific benefit: risk assessment models. The two main recommendations of the working group to the CHMP were to incorporate a structured list of benefit and risk criteria into the current CHMP assessment report template and to further explore methodologies for benefit-risk analysis, including a wide range of quantitative and semi-quantitative tools. A consequence of this was the initiation of the Benefit-Risk Methodology Project7, which eventually led to the European Medicines Agency (EMA) adopting the use of an Effects Table (ET) (see Figure 1) as a tool to summarise the key benefits and risks, by presenting a compact and consistent display of the data and uncertainties that are drivers of the decision.
Figure 1:
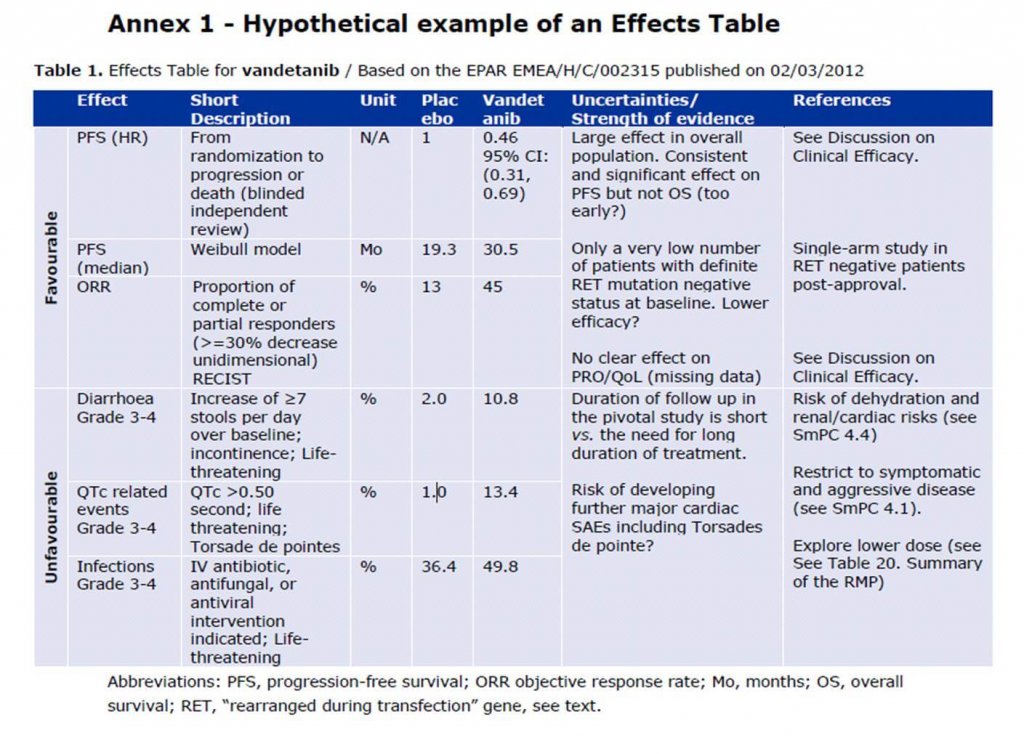
Despite the aforementioned project and the outcome for the EMA (other competent authorities use other quantitative tools), on the whole, quantitative benefit-risk assessment is not yet expected to replace qualitative evaluation and expert judgement is expected to remain the cornerstone of benefit-risk evaluation6. This is because, although it might be easy to have a computer calculate an overall benefit: risk score, some of the intricacies that the assessment is based on could be lost, and the statistical outcome could end up overriding good scientific judgment and basic common sense. Additionally, as indicated in good pharmacovigilance practices (GVP) Module XI – Signal Management8, whilst statistical methods may be appropriate for competent authorities and “Big Pharma” companies, the same methods may not be appropriate for small companies. It would, therefore, be difficult to implement a standard methodology. Furthermore, although the CIOMs Working Group IV guidance4 has now been around for 20 years, it is a useful guidance document to this day, as the principles are still relevant. This is evidenced by the way the values are interwoven into the GVP modules.
The CIOMs Working Group IV guidance4 recommends a standard outline for a written report to describe the results of a benefit-risk evaluation. The themes of which are briefly outlined below and can be applied to any pharmacovigilance document assessing risk, such as a signal validation report. In the main, the structure of the modern PBRER also follows this template. Firstly, the report should include an introduction comprising a brief description of the drug and in what countries it is marketed, the indications for use, identification of alternative therapies and a very brief description of the suspected or established major safety problem. Next, the benefit of the medicinal product should be established. To do this, the epidemiology and natural history of the target disease (indication) needs to be ascertained. This is key to any benefit: risk assessment as the greater the severity of the target disease, the more risks a patient will be willing to accept to be treated. In outlining the epidemiology of the disease, the incidence and prevalence should be considered, as well as whether the disease is self-limiting, intermittent, chronic or progressive. All of this can be deliberated to decide the patient’s prognosis and quality of life if left untreated. Another important aspect to include in the benefit assessment is the intended outcome of the treatment. For example, is the treatment second-line treatment (higher risks may be acceptable) or prophylaxis or diagnostic (potentially treating healthy individuals therefore high risks may be unacceptable). The author will also need to demonstrate the degree of efficacy achieved in clinical trials and effectiveness in clinical practice as well as stating alternative therapies, and if possible comparing the benefits of these therapies with the chosen medicine. For instance, if there is no alternative treatment available, then higher risks will be accepted with the medicine in question. Although, if there are safer alternatives e.g. lifestyle changes the reverse will be true. However, it should be noted that comparing benefits between alternative therapies can be difficult unless there are similar methods of data collection and analysis used.
Subsequently, the risks, specifically the newly identified risk, require analysis. This is done by presenting a detailed evaluation of data on the new risk along with probable and possible explanations. Where the data has originated from (i.e. case reports, case-control studies and large cohort studies) should also be considered to assess any limitations in the sources. The preventability, predictability, and reversibility of the risk, in association with the public health impact, should also be discussed. This is important as if the risk can be pre-empted, it is likely that the outcome of the assessment would be that additional risk minimisation measures can be applied, and the medicine can still be accessed by all the patients that require it. As above, alternative therapies need to be reviewed, but with respect to the risks; whether the same risk or equally severe occur with other available treatments, could determine the level of acceptability.
To conclude, the benefit: risk relationship should be summarised and all possible outcomes should be considered, before concluding which is most appropriate. This can be difficult, bearing in mind all of the above variables. In some situations, as discussed above, this can be achieved quantitatively, however, in many cases, the final assessment will be qualitative, or even subjective. In general, all available relevant data on benefits and risks should be assembled and presented in a transparent way. Briefly, outcomes can include: no action necessary, watch and wait (monitor), intensive additional data gathering, initiate new research, modify product and/or product information, restrict use, e.g. to subgroups, suspend product license, withdraw product, and communications. In terms of the outcome of the overall assessment, a review of the pros and cons and likely consequences of each action is required. Particularly, withdrawal of a product should only be considered if the balance of benefits and risks cannot be made favourable for the whole population or a sub-population. For example, through dose modification or contraindications. This is crucial, as withdrawing a medicinal product without proper deliberation of this can cause more harm than good through preventing patients from accessing a medicine. Safeguarding public health in this way, whilst minimising risks, is the root of benefit: risk assessments.
For more information, please contact bd@panacea.im
References